Disease is something few people love. There are exceptions though, particularly among those who appreciate the diversity and ecological role of plant disease. The early conservationist, Aldo Leopold, described the role of tree diseases in creating food and shelter for the animals on his farm in Wisconsin:
Soon after I bought the woods a decade ago, I realized that I had bought almost as many tree diseases as I had trees. My woodlot is riddled by all the ailments wood is heir to.
In October my grouse are often stuffed with oak galls… But for heart-rots, there would be no hollow oaks to furnish wild bees with oaken hives… My pileated woodpeckers chisel living pines, to extract fat grubs from the diseased heartwood. My barred owls find surcease from crows and jays in the hollow heart of an old basswood; but for this diseased tree their sundown serenade would probably be silenced.
The real jewel of my disease-ridden woodlot is the prothonotary warbler. He nests in an old woodpecker hole, or other small cavity, in a dead snag overhanging water. The flash of his gold-and-blue plumage amid the dank decay of the June woods is in itself proof that dead trees are transmuted into living animals, and vice versa. When you doubt the wisdom of this arrangement, take a look at the prothonotary.
Leopold, Aldo. 1949. “A Mighty Fortress.” In A Sand County Almanac & Other Writings on Ecology and Conservation, edited by Curt Meiner, 68-71. New York: Library of America.
I met Chris Preston, a Cambridgeshire botanist, when I was in the first year of my degree. We were at a botanical walk in one of the many fragments of ancient woodland that dot the landscape of southern England. He hadn’t just come for botany though – he was on the hunt for plant pathogens. I was amazed at how he conjured them up out of thin air, at their subtle beauty and how common they were. Since then I have started recording pathogens more systematically around Belfast, and have realised that they are far more diverse than I ever realised.

At the right time of year a walk around any urban area is guaranteed to show up at least 2 or 3 conspicuous pathogens. With some knowledge of which plants to check you can find 20 or 30 in an afternoon (walking around with Chris I realise just how much expert knowledge helps with finding inconspicuous species). The diversity of these organisms in many places begins to rival that of their hosts – and that’s just the ones you can see.
In fact, there are far more fungi living inside plants than will ever be visible to the naked eye. Ten or twenty leaf samples of a plant species can yield through genetic analysis over a hundred fungal species1! These fungi can be parasites (harming the plant), mutualists (benefiting the plant), or commensals (having no effect). Some fungi can help and hinder the plant at different stages in their life cycle.
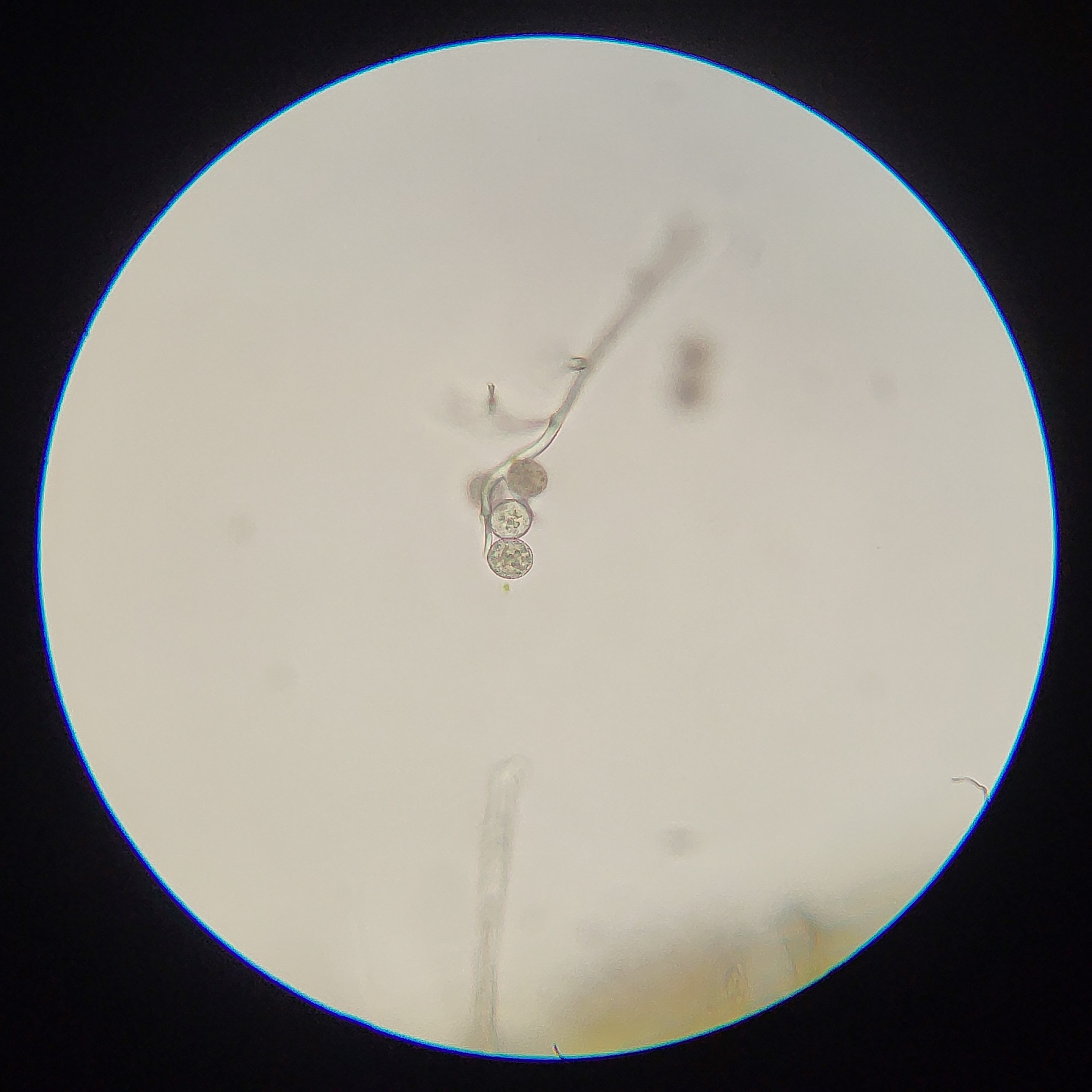
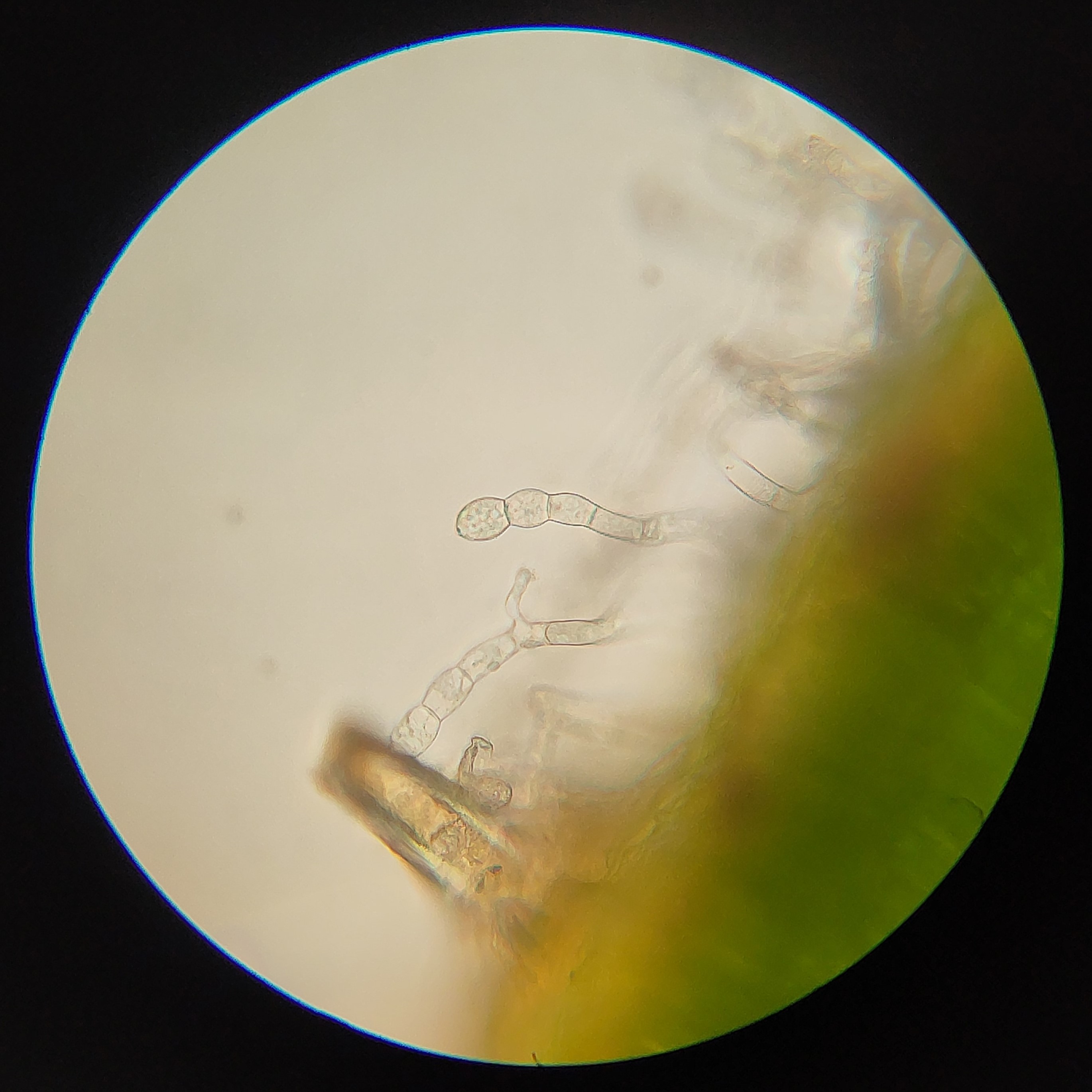
2: Conidiophores of Golovinomyces asperifolii, a fungal pathogen of Forget-Me-Not (Myosotis). In this case the rounded cells at the end are the conidia, and break off to disperse.
Although they may seem simple, unsophisticated organisms, many pathogens manipulate their hosts in complex and exacting ways. Biotrophs, pathogens which parasitise their host without killing its cells, must avoid triggering the plant’s defenses while extracting sustenance to grow and reproduce. A few rusts even manipulate the host’s development to force it to produce pseudoflowers, structures which attract insects that spread the fungus’s spores2. That pathogens can cause plants to produce novel structures for their own benefit is testament to their intricate coevolution with their hosts.

Besides their own diversity, diseases play a central role in the maintenance of plant biodiversity. For a long time it was a mystery why trees in tropical forests are so incredibly diverse, despite most of them being ecologically very similar. In the 1970s two ecologists, Janzen and Connell, independently explained this: species-specific diseases can prevent any single species from becoming dominant. As the density of a species increases, diseases specific to it spread more rapidly, and more young plants die or never germinate. This leads to an upper limit on how dominant any one species can be, creating gaps for other species and meaning many more trees can coexist than would otherwise.
The importance of disease in maintaining plant biodiversity becomes obvious when pathogens are removed. When fungicide is sprayed in an ecosystem, fungal diseases no longer limit the populations of plants. Diversity and evenness decrease, with groups like grasses and sedges benefiting from their freedom from disease and becoming more dominant. In an alpine grassland in China it has been shown that fungi are more important in this regard than oomycetes3.
Just as diseases of wild plants have existed inconspicuously for most of human history, they are also slipping away without much attention from conservationists. Their conservation status is rarely considered, but in Wales the smuts and allied fungi have been reasonably systematically surveyed and assessed, with a Red List published in 20184. 44% were found to be threatened or extinct, more than double that of any other assessed group of plants or fungi. In fact, their incredible specialisation on their plant hosts is their downfall, in a period of increasing habitat loss and homogenisation. Specialists are vulnerable to the extinction of their hosts – when the host is lost, its pathogens are too as a secondary extinction. More specialist species rely on fewer host species and so are more vulnerable to extinction5. Worse, pathogens are still vulnerable even if the host does not go extinct. Most pathogens do not infect every host; their population fluctuates. This means if host populations fall below some threshold the pathogen can go extinct simply through random chance, as none of the available host plants happen to be infected (stochastic extinction).
Even species on common hosts can be surprisingly rare: Lesser Celandine (Ficaria verna) hosts a leaf smut, Urocystis ficariae, with just a few tens of records from Britain (it is not known from Ireland but may well be present). Other pathogens that infect this species, like Entyloma ficariae and Uromyces ficariae, are nearly ubiquitous. It is hard to see why something which infects such a common host should be so rare but the problem of conserving it remains. Could even a moderate decline in Ficaria put Urocystis at risk of stochastic extinction? These are the consequences of declines in common species which we rarely consider.
Beyond fascination in their endless diversity, my interest in plant pathogens has taken me into community ecology, the study of how sets of species assemble and change over time and space. I want to look at how different habitats support different pathogen species, even when the same hosts are present. In particular I think ancient and recently created grasslands are likely to differ substantially – it may take many years for pathogen communities to form, and if a host is relatively rare it may be very difficult for pathogens to disperse to newly created habitat. Could this mean the maintenance of plant diversity by pathogens is weaker in younger grasslands?
I hope to inspire other people to pay more attention to these maligned, ignored organisms. I think they are really special and deserve to be recognised as such. The clearest indication of how overlooked they are is how easy it is to find new species to Ireland – I have found at least three in just a few weeks during this Easter holiday. I leave you with a picture of Albugo leimonios on Cuckooflower (Cardamine pratensis), which is currently my favourite pathogen-host combination.

References
- G. S. Gilbert, I. M. Parker, “Community ecology” in The Evolutionary Ecology of Plant Disease, 1st Ed., (Oxford University Press, 2023), pp. 203–222.
- B. A. Roy, Floral mimicry by a plant pathogen. Letters to Nature 362 (1993).
- X. Liu, I. M. Parker, G. S. Gilbert, Y. Lu, Y. Xiao, L. Zhang, M. Huang, Y. Cheng, Z. Zhang, S. Zhou, Coexistence is stabilized by conspecific negative density dependence via fungal pathogens more than oomycete pathogens. Ecology 103 (2022).
- R. G. Woods, A. O. Chater, P. A. Smith, R. N. Stringer, D. A. Evans, Smut and Allied Fungi of Wales: A Guide, Red Data List and Census Catalogue = Y Gwir-Barddu a Ffyngau Perthynol Cymru : Arweiniad, Rhestr Ddata Coch a Chatalog Cyfrifiad (A.O. Chater, Aberystwyth, 2018).
- K. D. Lafferty, Biodiversity loss decreases parasite diversity: theory and patterns. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 2814–2827 (2012).

Leave a Reply